Nuvaxovid
Company Novavax should not be given to individuals. 2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US.

Novavax Covid 19 Vaccine Nuvaxovid Approved By Mhra Gov Uk
After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further.
. Company Novavax should not be given to individuals. Stockholm Nov 3 Nuvaxovid the Covid-19 vaccine created by US company Novavax should not be given to individuals younger than 30 years the Public Health Agency of. Beslutet är temporärt och gäller från.
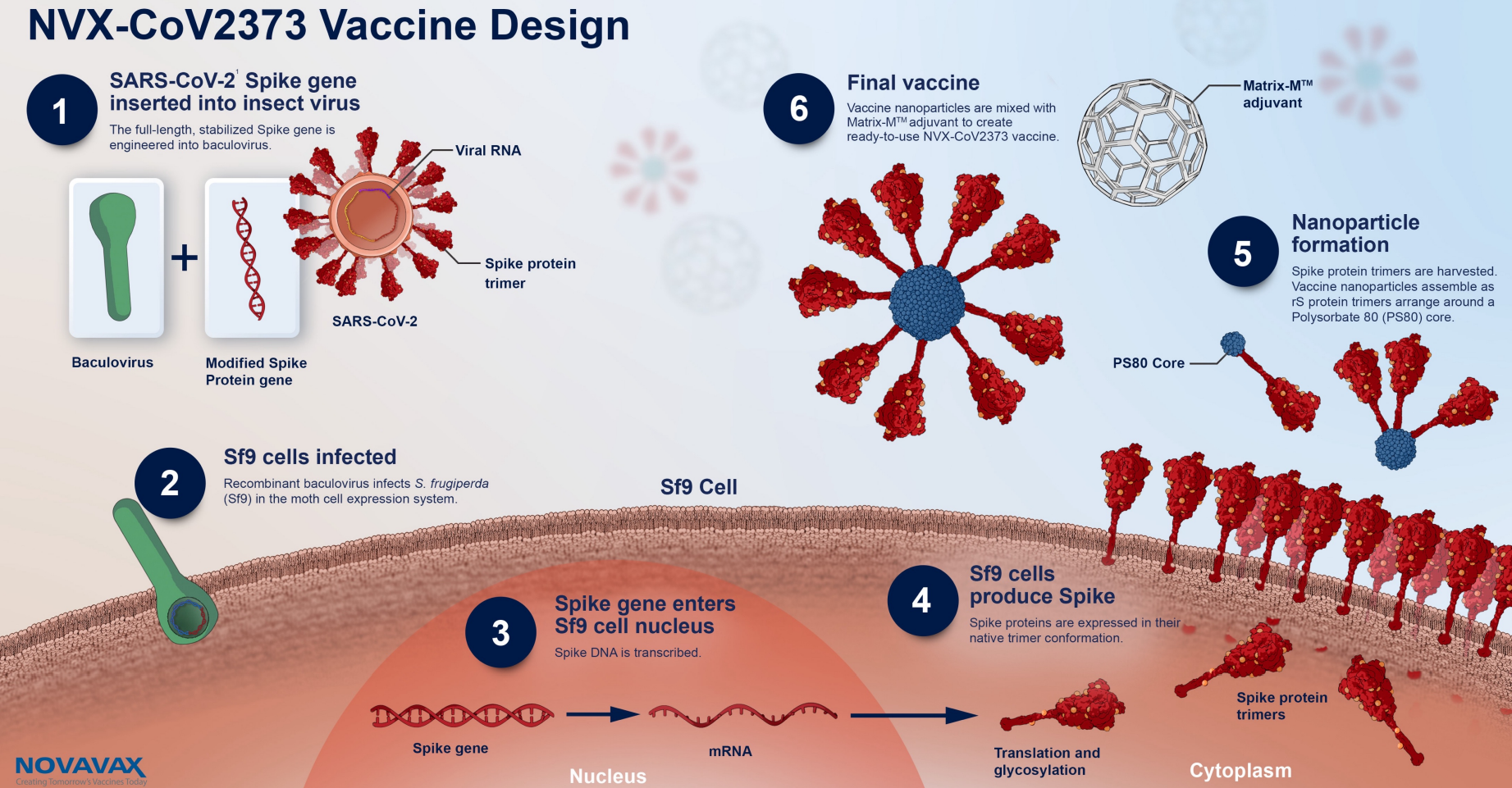
Nu stoppar Folkhälsomyndigheten användningen bland personer. Nyheter 02 nov 2022 Folkhälsomyndigheten pausar användningen av covidvaccinet Nuvaxovid hos personer under. Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach. On December 20 2021 the. This vaccine is currently being used in Sweden and as of date a total of 7000.
18 hours agoPublicerad idag 0702. 17 hours agoStopp för vaccination med Nuvaxovid för de under 30. Name of the medicinal product.
37 minutes agoThe US company Novavax came up with another vaccine to fight the virus - Nuvaxovid. The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. Qualitative and quantitative composition.
Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista.
This is a multidose vial. 15 hours ago3rd November 2022 0355 GMT11. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19.
The Summary of Product Characteristics is a description of a. Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic.
Find detailed technical information such as the product monograph and. COVID-19 Vaccine recombinant adjuvanted 2. Det eftersom att data.
17 hours agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant. Clinical trials showed that the vaccine has around 90 efficacy.
Nuvaxovid-rokote sopii lähes kaikille aikuisille. Nuvaxovid dispersion for injection. The Novavax Nuvaxovid COVID-19 vaccine was authorized for use in Canada under the Food and Drug Regulations.
HSAs assessment is that although the. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten.
Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022. Rokotteesta ei myöskään ole haittaa vaikka. As such HSA will be monitoring the incidence rate of pericarditis or inflammation of the outer lining of the heart and myocarditis.
The addition of the saponin-based.

Is The Novavax Covid Vaccine Worth The Hype Medpage Today

Ec Expands Novavax Nuvaxovid Covid 19 Vaccine Approval

Vaccine Against Coronavirus Nuvaxovid Novavax Niph
Nuvaxovid Otazky A Odpovedi K Vakcine Od Spolecnosti Novavax Kurzy Cz

Cdc Endorses Novavax Covid Shot For Adults Fortune

Nuvaxovid Covovax Novavax Vaccine Covid 19 Info Vaccines

Ministry Of Health Singapore On Instagram Registration For The Nuvaxovid Vaccine By Novavax Has Begun Individuals Aged 18 Years And Above May Receive The Vaccine For Their Primary
Tga Investigates Possible Myocarditis Link To Nuvaxovid Ausdoc

Novavax Nuvaxovid Covid 19 Vaccines Will Also Be Available From Rauma Healthcare Services In The Future Rauma Fi

Fda Signs Off On Novavax Covid 19 Shot For Booster Use
News Conditional Marketing Authorisation Application Submitted For Novavax S Covid 19 Vaccine Nuvaxovid Paul Ehrlich Institut

Ema Recommends Nuvaxovid For Authorisation In The Eu Certifico Srl
Novavax Nuvaxovid Covid 19 Vaccine Approved In South Korea For Use In Adolescents Aged 12 Through 17 World Pharma Today

European Union Authorizes Novavax Booster
Novavax Covid 19 Vaccine Nuvaxovid Provisionally Registered In Australia As A Booster In Individuals Aged 18 And Over Pharmtech Focus

Nvx Cov2373 Recombinant Adjuvanted Covid 19 Vaccine
New Protein Based Covid 19 Vaccine Could Help Boost Rates Say Pharmacists Cbc News

My Kingdom For A Booster Shot Hiccups Are Yet Another Vaccine Trial Hassle Medpage Today
